| |
| Gasification |
| Enhanced Gasification; TurnW2E™ |
W2E has developed waste-to-energy technology for utilizing a
variety of waste materials to make renewable and alternative
energy products. The technology can process virtually any
carbonaceous material, converting it into forms of usable energy
that can be consumed or sold easily.
At the core of the W2E technology is a process known as
gasification. It is a well-known technology for converting materials
into a clean-burning synthesis gas, which is then combusted for
power production, or further processed to produce hydrogen for
transportation fuels, or ammonia for use in fuel cells or as fertilizer.
The history of gasification process goes back many decades. There
is significant experience with wood gasification at various system
sizes, and with coal gasification, at relatively large applications.
The W2E technology has incorporated the best elements of past
gasification designs and performances to yield a very flexible and
reliable waste-to-energy system. |
| |
| WHAT IS GASIFICATION? |
| |
Gasification converts any carbon-containing material into a
synthesis gas (syngas). The syngas is a combustible gas mixture,
sometimes known as ‘producer gas’, typically contains carbon
monoxide, hydrogen, nitrogen, carbon dioxide and methane. The
syngas has a relatively low calorific value, ranging from 100 to 300
BTU/SCF. The syngas can be used as a fuel to generate electricity
or steam. Alternatively, it can be used as a basic chemical building
block for a large number of applications in the petrochemical and
refining industries.
The overall thermal efficiency of gasification process is more than
75%. Gasification can accommodate a wide variety of gaseous,
liquid, and solid feed stocks and it has been widely used in
commercial applications for more than 50 years in the production
of fuels and chemicals. Conventional fuels such as coal and oil,
as well as low- or negative-value materials and wastes such as
petroleum coke, heavy refinery residuals, secondary oil-bearing
refinery materials, municipal sewage sludge, hydrocarbon
contaminated soils, and chlorinated hydrocarbon byproducts have
all been used successfully in gasification operations. |
| |
| CHEMICAL REACTION OF GASIFICATION |
| |
The chemical reactions in gasification process take place in the presence of steam in an oxygen-lean,reducing atmosphere. The ratio of oxygen molecules to carbon molecules is far less than one in the gasification reactor.
A portion of the fuel undergoes partial oxidation by precisely controlling the amount of oxygen fed to the gasifier. The heat released in the first reaction provides the necessary energy for the other gasification reaction to proceed very rapidly. In the Turn W2E™ system, gasification temperatures and pressures within the refractory-lined reactor typically range from 800 Deg C to 1200 Deg C and near atmospheric pressure to few inches of water respectively. |
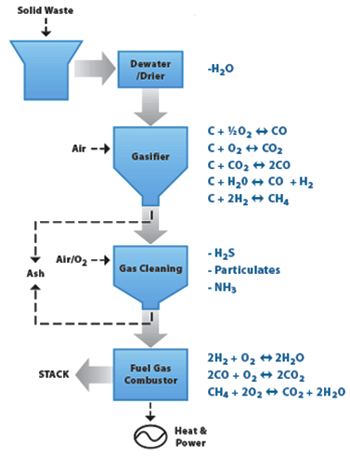 |
At higher temperatures the endothermic reactions of carbon with steam are favored. A wide variety of carbonaceous feed stocks can be used in the gasification process. Low-BTU wastes may be blended with high - BTU supplementary fuels such as coal or petroleum coke to maintain the desired gasification temperatures in the reactor.
The reducing atmosphere within the gasification reactor prevents
the formation of oxidized species such as SO2 and NOx which are replaced by H2S (with lesser amounts of COS), ammonia, and nitrogen (N2). These species are much easier to scrub from the syngas than their oxidized counterparts before the syngas is utilized for power. |
|
| |
| GASIFICATION VS. INCINERATION |
| |
While gasification and incineration are both thermal processes,
it is important to point out the advantages of gasificati on over
incineration. Incineration is simply a mass burn technology with heat recovery to produce steam and/or electricity. It has negative
connotations because during the direct combustion of the waste,
dangerous carcinogenic compounds such as dioxins and furans are
formed, which are discharged into the atmosphere. In contrast,
gasification employs the conversion of waste into syngas, which can
then be used for generating steam and/or electricity, for producing
chemicals for high-value products, or for producing liquid fuels. |
| |
| History |
| 1850-1940 |
• To produce “town
gas” for light & heat
• Gasification of coa
- All gas for fuel and
light |
| 1940-1975 |
• To produce synthetic
fuel
• To produce liquid
fuels and chemicals |
| 1975-1990 |
• First Integrated
Gasification
Combined Cycle
(IGCC) electric
power plant |
| 1990-2000 |
• US agencies
provided fi nancial
support for IGCC
process |
| 2000-Present |
• Turnkey thermal & power
Green house gas from
biomass
• Renewed focus on
reducing GHG emissions
• Biomass to liquid
fuel conversion
commercialized |
| |
|
|
The synthesis gas is produced under controlled conditions, and
is generated without the formation of impurities associated with
incinerator flue gas. Gasification emissions are generally an order
of magnitude lower than the emissions from an incinerator. |
| |
| Key differences between Gasification and Incineration |
| INCINERATION |
W2E Gasification |
| Combustion Vs. Gasification |
Designed to maximize the
conversion of waste to CO2 and
H2O |
Designed to maximize the conversion of
waste to CO and H2 |
Employs large quantities of
excess air |
Operates under controlled amount of air |
| Highly oxidizing environment |
Reducing environment |
| Gas Cleanup |
| Treated flue gas discharged to
atmosphere. Flue gas contains
dioxins and furans |
Cleaned syngas used for chemical
production and / or power production
(with subsequent clean flue gas
discharge) |
| Fuel sulfur converted to SOx and
discharged with flue gas |
Recovery of reduced sulfur species in
the form of a high purity elemental sulfur
or sulfuric acid byproduct is feasible |
| Residue and Ash Slag Handling |
| Bottom ash and fly ash collected
and disposed as waste |
Bottom ash and fl y ash collected and
disposed of as waste |
|
| |
GASIFICATION FEEDSTOCK |
| |
There are many carbonaceous materials that are suitable for
gasification. These include wood, paper, peat, lignite, coal,
including coke derived from coal, saw dust and agro-residues. All
of these solid fuels are composed primarily of carbon with varying
amounts of hydrogen, oxygen, and impurities, such as sulfur, ash, and moisture.Municipal Solid Waste (MSW) is also a good
candidate for gasification; however, it poses a special challenge
for waste processors, due its non-homogenous characteristics, high moisture content and unpredictable calorific value. |
 |
| |
|
W2E has
overcome this challenge by designing a unique gasifier.
Thus, the TurnW2E™ gasification process presents a new and better
method for the treatment of non-homogenous waste streams.
Gasification is fast becoming a favored technology for recovering
energy from MSW and other solid wastes, and the TurnW2E™
system stands ready to provide this service to the industry.
|
| |
GASIFICATION TECHNOLOGIES |
| |
Moving Bed: The fuel is dry-fed through the top of a reactor onto
a bed – usually a slow-moving metal grate. As the fuel descends,
it reacts with gasifying agents (steam and oxygen) flowing in a
counter-current through the bed. The syngas has a low temperature
(400-500 Deg C) and contains significant quantities of tars and oils.
Entrained Flow: The fuel and gasifying agents flow in the same
direction (and at rates in excess of other gasifier types). The
feedstock – which may be dry-fed (mixed with nitrogen) or wet-fed
(mixed with water) – goes through the various stages of gasification
as it moves with the steam and oxygen flow.
|
 |
|
Fluidized Bed: The fuel, introduced into an upward flow of steam/
oxygen, remains suspended in the gasifying agents while the
gasification process takes place.
Rotary Reactor: Gasifying agents, air and/or oxygen and steam are
introduced along a rotating horizontal cylindrical reactor vessel.
Gasification takes place along the length of the vessel in stages
until SynGas is released from the end while ash drops out. Rotary
reactors, such as the TurnW2E(TM) developed by W2E, enable
complete mixing of the gasifying agents with air while the process
is closely controlled by the rotational speed and air flow. The lower
gas temperatures (800 - 900 Deg C) - while high enough to volatilize tar
and oils – allows easier handling of ash. |
| |
|
|
|
|
|

